l Different types of radiation
Most of the nuclei are stable, due to the strong force bonding the protons and neutrons together. However, some nuclei, usually large nuclei like Uranium-235, are unstable. In order to return to their stable state, they may go through a process called “decay”, which may give out particles or electromagnetic waves as by-products. We say these unstable nuclei are radioactive and they are named as radionuclides. The particles and electromagnetic waves released are called radiation.
 Non-ionizing radiation is the electromagnetic wave with relatively low energy level. It can only cause the molecules to vibrate and induces heating effects. Non-ionizing radiation includes ultraviolet, visible light, infrared, microwave as well as radio wave.
Non-ionizing radiation is the electromagnetic wave with relatively low energy level. It can only cause the molecules to vibrate and induces heating effects. Non-ionizing radiation includes ultraviolet, visible light, infrared, microwave as well as radio wave.
Ionizing radiation is high energy radiation which is able to remove electrons from atoms, resulting in the formation of ions. Ions carry positive charges and are readily for chemical reactions. Ionizing radiation is more dangerous in the sense that it can cause the breakdown of a substance. Ionizing radiation can be high speed particles like alpha particles, beta particles as well as neutrons; or high energy electromagnetic waves like gamma rays and x-rays.
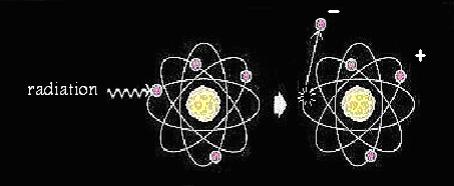
↑ An atom is being ionized.
Ø Alpha particles (α-particles)
α-particles are particles consisting of two protons and two neutrons, which are identical to Helium-4 nuclei. They are generally emitted by heavy atoms, i.e. atoms having atomic numbers greater than 82, through a process called “alpha decay”. They have relatively large masses, low velocities and with +2 charges. These make them very likely to interact with other atoms and lose their energy quickly. As a result, the ionizing power of α-particles is very strong, but their penetrating power is the weakest among all types of ionizing radiation. α-particles can be stopped easily by a cardboard.
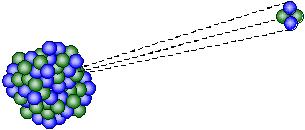
↑ α-decay
Ø Beta particles (β-particles)
In general, β-particles refer to high speed electrons. They are emitted through “beta decay” of some radioactive nuclei. They carry -1 charges and are affected by an electromagnetic field. Their ionizing power is weaker than that of α-particles. Since they have much smaller masses and higher velocities than α-particles, β-particles have a greater penetrating power. A sheet of aluminium a few millimeters thick is enough to stop them.
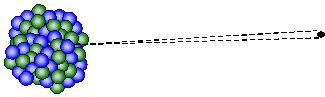
↑ β-decay
Ø Gamma rays (γ-rays)
γ-rays are a type of high energy electromagnetic waves. They are generated by the transitions within radioactive nuclei, which is also known as “gamma decay”. γ-rays have no mass and no electrical charge. They are not affected by an electromagnetic field. γ-rays have the highest frequency, the shortest wavelength, and thus, the highest energy within the electromagnetic spectrum. Although their ionizing power is weak, they have great penetrating power and can pass through human body. Serious damages can be caused when they pass through living cells. γ-rays cannot be stopped completely, but thick barriers of lead or concrete can effectively diminish them.
Ø X-rays
X-rays are also a type of high energy electromagnetic waves, just like γ-rays. In fact, the properties of the two types of rays are quite similar. The only difference is their origins. Unlike γ-rays, x-rays are emitted from the electron cloud as a result of electron excitation.

↑ A comparison between the origins of γ-rays and X-ray.
Ø Neutrons
Neutrons are constituents of the atomic nucleus. They carry no charge and have a mass which is a little bit higher than that of protons. Neutron radiation is a kind of ionizing radiation which consists of free neutrons. They can be produced by nuclear fission or fusion processes. Although they are neutral, their ionizing power is still strong. Fast-moving neutrons are very penetrating and can only be stopped by hydrogen-rich materials, such as water or paraffin. In nuclear reactors, water is commonly used to moderate and control the speed of neutrons.