In a typical fission process in a nuclear power plant, an incoming neutron hits a uranium 235 atom to produce two lighter fission products together with some energy in the form of nuclear radiation and between 2 to 3 neutrons for further fission reactions.
Ø Types of Ionizing Radiation
1. Alpha particles – swiftly moving nuclei of helium atoms with positive charges.
2. Beta particles – high speed electrons with more penetrating power than alpha particles.
3. X-rays and gamma rays – penetrating rays passing through body.
4. Neutrons – carrying no charge but with high penetrating power.
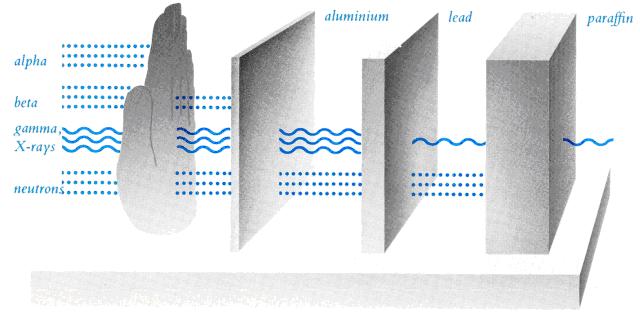
Common Types of Ionizing Radiation
Image Credit: Government Information Services, HKSARG
Ø Sources of radiation
There are two generic sources of radiation – natural and artificial. Natural radiation includes cosmic ray from space and radiation emitted by radioactive substances that exist on food and habitat. Artificial radiation comes from radioactive fallout from nuclear testing, x-rays emissions from vacuum tubes, such as medical diagnostic machine, television and video display units, and use of radioactive materials in consumer products such as smoke detectors.