l What is “Nuclear Physics”?

Nuclear Physics is a branch of Physics that studies the structures, properties and interactions of atomic nuclei. Atoms are the tiny particles of which all the substances in the world are composed. The structure of an atom is just like our solar system: in the center, there is a “sun” named as “nucleus”, which occupies most of the system’s mass; there are some “planets” called “electrons”, orbiting round the center. Nuclear Physics is basically a subject focusing on the research of the nucleus, and developing the possible applications in our society.
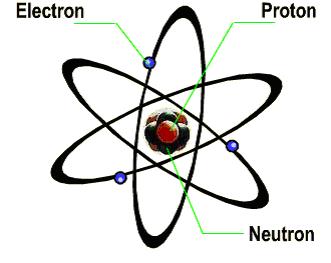
l Structure of atoms
A nucleus is generally composed of two kinds of particles: protons and neutrons, except for hydrogen nucleus, which only contains a proton. Protons carry positive charges while neutrons are neutral. Electrons are negatively charged and the number of electrons is usually equal to that of protons. Thus, an atom is usually neutral as a whole. Protons and neutrons have similar masses, but electrons have a much smaller masses. Therefore, when we talk about the mass of an atom, the masses of the electrons inside are often neglected.
l Origin of radioactivity
Most of the nuclei are stable, due to the strong force bonding the protons and neutrons together. However, some nuclei, usually large nuclei like Uranium-235, are unstable. In order to return to their stable state, they may go through a process called “decay”, which may give out particles or electromagnetic waves as by-products. We say these unstable nuclei are radioactive and they are named as radionuclides. The particles and electromagnetic waves released are called radiation.

|