l Fusion and Fission
“Fusion” means “joining up”, while “fission” means “splitting”. Nuclear fusion and nuclear fission are two different types of nuclear activities which both release large amount of energy.
Nuclear fusion is a process which smaller nuclei join together to form larger ones. Usually, small atoms like hydrogen and helium undergo this process. In this way, energy is given out and the atoms would become more stable. Our Sun is giving out large amount of light and heat seconds by seconds through this process.
Nuclear fission is a process which heavy nuclei are split into parts. For atoms having atomic number greater than 82, their nuclei are unstable and are spontaneously decaying to form more stable nuclei. The total number of the unstable nuclei is continually decreasing; radiation, neutrons and lighter nuclei are then given out. For a fixed amount of unstable nuclei, say, Carbon-14, scientists discovered that its total amount is deducted by half for every 5730 years, so 5730 years was said to be the “half-life” of Carbon-14.
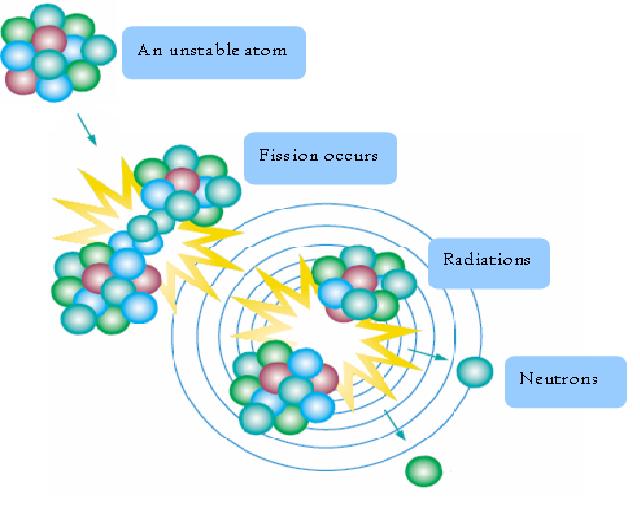
Besides, nuclear fission can also be triggered as well. Some atoms like Uranium-235 are highly unstable. When one of these atoms is hit by an incoming neutron, the atom will be split apart, heat and 2 to 3 neutrons are released. The neutrons released will then split the nuclei of the neighbouring atoms. The spitting continues and this is called a “chain reaction”. In this way, a lot of heat can be accumulated at a very short time.
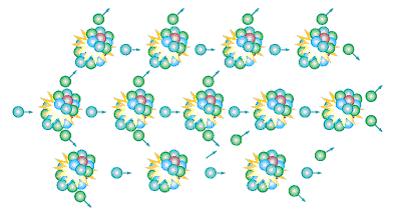
↑ Chain reaction
One point to note, for atoms having atomic number smaller than 82, they cannot undergo nuclear fission; they can only carry out nuclear fusion.